Na-ion mobility in P2-type Na0.5MgxNi0.17−xMn0.83O2 (0 ≤ x ≤ 0.07) from electrochemical and muon spin relaxation studies†
Abstract
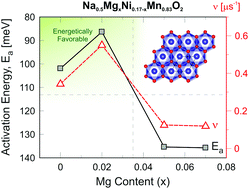
Sodium transition metal oxides with a layered structure are one of the most widely studied cathode materials for Na+-ion batteries. Since the mobility of Na+ in such cathode materials is a key factor that governs the performance of material, electrochemical and muon spin rotation and relaxation techniques are here used to reveal the Na+-ion mobility in a P2-type Na0.5MgxNi0.17−xMn0.83O2 (x = 0, 0.02, 0.05 and 0.07) cathode material. Combining electrochemical techniques such as galvanostatic cycling, cyclic voltammetry, and the galvanostatic intermittent titration technique with μ+SR, we have successfully extracted both self-diffusion and chemical-diffusion under a potential gradient, which are essential to understand the electrode material from an atomic-scale viewpoint. The results indicate that a small amount of Mg substitution has strong effects on the cycling performance and the Na+ mobility. Amongst the tested cathode systems, it was found that the composition with a Mg content of x = 0.02 resulted in the best cycling stability and highest Na+ mobility based on electrochemical and μ+SR results. The current study clearly shows that for developing a new generation of sustainable energy-storage devices, it is crucial to study and understand both the structure as well as dynamics of ions in the material on an atomic level.
- This article is part of the themed collection: Energy Frontiers: Electrochemistry and Electrochemical Engineering


 Please wait while we load your content...
Please wait while we load your content...