Photoelectron photofragment coincidence spectroscopy of aromatic carboxylates: benzoate and p-coumarate†
Abstract
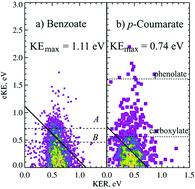
Photoelectron–photofragment coincidence spectroscopy was used to study the dissociation dynamics of the conjugate bases of benzoic acid and p-coumaric acid. Upon photodetachment at 266 nm (4.66 eV) both aromatic carboxylates undergo decarboxylation, as well as the formation of stable carboxyl radicals. The key energetics are computed using high-level electronic structure methods. The dissociation dynamics of benzoate were dominated by a two-body DPD channel resulting in CO2 + C6H5 + e−, with a very small amount of stable C6H5CO2 showing that the radical ground state is stable and the excited states are dissociative. For p-coumarate (p-CA−) the dominant channel is photodetachment resulting in a stable radical and a photoelectron with electron kinetic energy (eKE) <2 eV. We also observed a minor two-body dissociative photodetachment (DPD) channel resulting in CO2 + HOC6H4CHCH + e−, characterized by eKE <0.8 eV. Evidence was also found for a three-body ionic photodissociation channel producing HOC6H5 + HCC− + CO2. The ion beam contained both the phenolate and carboxylate isomers of p-CA−, but DPD only occurred from the carboxylate form. For both species DPD is seen from the first and second excited states of the radical, where vibrational excitation is required for decarboxylation from the first excited radical state.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...