Oxo-sulfido molybdenum and tungsten fluorides with M–O and M–S multiple bonds†
Abstract
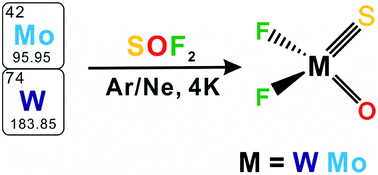
Oxo-sulfido molybdenum/tungsten difluorides in the form of Mo(O)(S)F2 and W(O)(S)F2 were prepared in cryogenic matrices via the reactions of laser-ablated metal atoms and SOF2. Both complexes were characterized to possess one oxo, one sulfido and two fluoro ligands terminally bound to the metal center according to the results of infrared spectroscopy combined with isotopic substitution, and non-planar Cs symmetries with closed shell singlet ground states were established on the basis of density functional calculations. The SMoO and SWO bond angles of Mo(O)(S)F2 and W(O)(S)F2 are around 107°, which are close to those of bent MoO22+ and WO22+ (∼101°). Natural bond orbital calculations indicate the presence of a Mo/W–O double bond in Mo(O)(S)F2 and W(O)(S)F2 while the Mo/W–S bond is better described as a triple bond upon F− coordination to SMoO2+ and SWO2+. UV-Vis irradiation is required in order to form the oxo-sulfido molybdenum/tungsten difluorides when metal atoms react with SOF2 in cryogenic matrices.


 Please wait while we load your content...
Please wait while we load your content...