The enhanced dissociation and associated surface structure of the anesthetic propofol at the water interface: vibrational sum frequency generation study†
Abstract
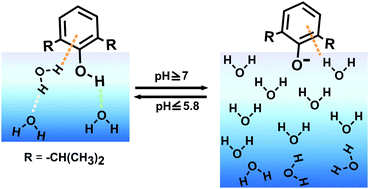
Propofol, the most administered drug for general anesthesia, affects the acid–base equilibrium at the interfacial region of arterial blood. Hence, the structure of propofol at the water interface under different pH conditions has been measured using the surface-selective vibrational sum frequency generation (VSFG) technique to understand the hydration as well as the dissociation of propofol at the water interface. Propofol remains in its neutral form at pH ≤ 5.8 in which the OH group of propofol forms a hydrogen bond with interfacial water molecules, where a few interfacial water molecules also interact with the π electron density of propofol. By contrast, propofol prefers to be in the deprotonated state at pH ≥ 7, due to which the surface of water becomes negatively charged and hence the interfacial water becomes oriented and the intensity of the OH stretch of water is enhanced. The pKa of propofol at the water interface is ∼three units lower than in the bulk medium indicating that the dissociation of propofol is notably enhanced at the water interface. These VSFG studies suggest that, unlike the bulk, propofol prefers to be in the charged state at the water interface under physiological conditions, which may be important in understanding its diffusion and acid–base equilibrium in the interfacial arterial blood region.


 Please wait while we load your content...
Please wait while we load your content...