Water and oxygen co-induced microstructure relaxation and evolution in CH3NH3PbI3†
Abstract
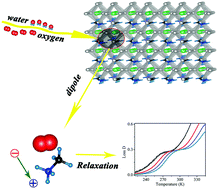
Owing to perovskite possessing the outstanding optoelectronic properties, perovskite-based solar cells show prominent performance. The stability of perovskite-based solar cells hampers the progress of commercialization, so it is important to understand the microstructure mechanism of perovskite degradation under the humidity and oxygen environmental conditions. In this study, a meaningful Debye-type dielectric relaxation was observed under water vapor and oxygen co-treatment conditions. Interestingly, the relaxation was not observed under water vapor or oxygen treatment individually. This new dielectric relaxation is identified as a direct result of dipole jump, and its activation energy was measured to be 630 ± 6 meV. According to photoelectron spectroscopy and 13C nuclear magnetic resonance data, we suggest that the dipoles are formed by CH3NH3+ (MA+) and superoxide (O2−), which originate from the distorted crystal lattice and water vapor-weakened hydrogen bonds of Pb–I cages. In addition, the activation energy fitted by dielectric relaxation might be the energy of ion migration. This study contributes to understanding the mechanism of perovskite degradation from the view of microstructure relaxation and evolution, and also provides a method for the analysis of ion migration energy.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...