Spontaneous NaCl-doped ices Ih, Ic, III, V and VI. Understanding the mechanism of ion inclusion and its dependence on the crystalline structure of ice†
Abstract
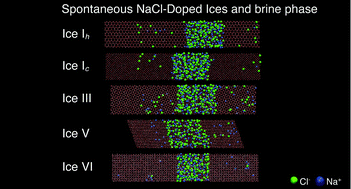
Direct coexistence simulations on a microsecond time scale have been performed for different types of ice (Ih, Ic, III, V, and VI) in contact with a NaCl aqueous solution at different pressures. In line with the previous results obtained for ice Ih [Conde et al., Phys. Chem. Chem. Phys., 2017, 19, 9566–9574], our results reveal the spontaneous growth of a new ice doped phase and the formation of a brine rejection phase in all ices studied. However, both the preferential incorporation of ions into the ice lattice and the inclusion mechanisms depend on the crystalline structure of each ice. This work shows the inclusion of Cl− and Na+ ions in ice from salt using molecular dynamics simulation, in agreement with the experimental evidence found in the literature. The model used for water is TIP4P/2005. For NaCl we employ a set of potential parameters that uses unit charges for the ions.


 Please wait while we load your content...
Please wait while we load your content...