Hofmeister effects on protein stability are dependent on the nature of the unfolded state†
Abstract
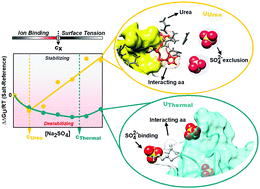
The interpretation of a salt's effect on protein stability traditionally discriminates low concentration regimes (<0.3 M), dominated by electrostatic forces, and high concentration regimes, generally described by ion-specific Hofmeister effects. However, increased theoretical and experimental studies have highlighted observations of the Hofmeister phenomena at concentration ranges as low as 0.001 M. Reasonable quantitative predictions of such observations have been successfully achieved throughout the inclusion of ion dispersion forces in classical electrostatic theories. This molecular description is also on the basis of quantitative estimates obtained resorting to surface/bulk solvent partition models developed for ion-specific Hofmeister effects. However, the latter are limited by the availability of reliable structures representative of the unfolded state. Here, we use myoglobin as a model to explore how ion-dependency on the nature of the unfolded state affects protein stability, combining spectroscopic techniques with molecular dynamic simulations. To this end, the thermal and chemical stability of myoglobin was assessed in the presence of three different salts (NaCl, (NH4)2SO4 and Na2SO4), at physiologically relevant concentrations (0–0.3 M). We observed mild destabilization of the native state induced by each ion, attributed to unfavorable neutralization and hydrogen-bonding with the protein side-chains. Both effects, combined with binding of Na+, Cl− and SO42− to the thermally unfolded state, resulted in an overall destabilization of the protein. Contrastingly, ion binding was hindered in the chemically unfolded conformation, due to occupation of the binding sites by urea molecules. Such mechanistic action led to a lower degree of destabilization, promoting surface tension effects that stabilized myoglobin according to the Hofmeister series. Therefore, we demonstrate that Hofmeister effects on protein stability are modulated by the heterogeneous physico-chemical nature of the unfolded state. Altogether, our findings evidence the need to characterize the structure of the unfolded state when attempting to dissect the molecular mechanisms underlying the effects of salts on protein stability.


 Please wait while we load your content...
Please wait while we load your content...