An experimental and master-equation modeling study of the kinetics of the reaction between resonance-stabilized (CH3)2CCHCH2 radical and molecular oxygen†
Abstract
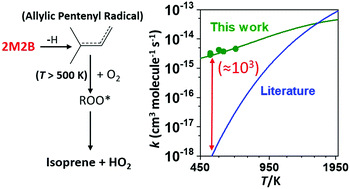
The kinetics of the reaction between resonance-stabilized (CH3)2CCHCH2 radical (R) and O2 has been investigated using photoionization mass spectrometry, and master equation (ME) simulations were performed to support the experimental results. The kinetic measurements of the (CH3)2CCHCH2 + O2 reaction (1) were carried out at low helium bath-gas pressures (0.2–5.7 Torr) and over a wide temperature range (238–660 K). Under low temperature (238–298 K) conditions, the pressure-dependent bimolecular association reaction R + O2 → ROO determines kinetics, until at an intermediate temperature range (325–373 K) the ROO adduct becomes thermally unstable and increasingly dissociates back to the reactants with increasing temperature. The initial association of O2 with (CH3)2CCHCH2 radical occurs on two distinct sites: terminal 1(t) and non-terminal 1(nt) sites on R, leading to the barrierless formation of ROO(t) and ROO(nt) adducts, respectively. Important for autoignition modelling of olefinic compounds, bimolecular reaction channels appear to open for the R + O2 reaction at high temperatures (T > 500 K) and pressure-independent bimolecular rate coefficients of reaction (1) with a weak positive temperature dependence, (2.8–4.6) × 10−15 cm3 molecule−1 s−1, were measured in the temperature range of 500–660 K. At a temperature of 501 K, a product signal of reaction (1) was observed at m/z = 68, probably originating from isoprene. To explore the reaction mechanism of reaction (1), quantum chemical calculations and ME simulations were performed. According to the ME simulations, without any adjustment to energies, the most important and second most important product channels at the high temperatures are isoprene + HO2 (yield > 91%) and (2R/S)-3-methyl-1,2-epoxybut-3-ene + OH (yield < 8%). After modest adjustments to ROO(t) and ROO(nt) well-depths (∼0.7 kcal mol−1 each) and barrier height for the transition state associated with the kinetically most dominant channel, R + O2 → isoprene + HO2 (∼2.2 kcal mol−1), the ME model was able to reproduce the experimental findings. Modified Arrhenius expressions for the kinetically important reaction channels are enclosed to facilitate the use of current results in combustion models.


 Please wait while we load your content...
Please wait while we load your content...