Theoretical study of spodium bonding in the active site of three Zn-proteins and several model systems†
Abstract
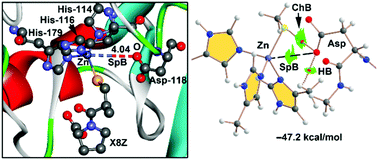
In this manuscript, three examples retrieved from the PDB are selected to demonstrate the existence and relevance of spodium bonding (SpB) in biological systems. SpB is defined as an attractive noncovalent interaction between elements of group 12 of the periodic table acting as a Lewis acid and any atom or group of atoms acting as an electron donor. The utilization of this term (SpB) is convenient to differentiate classical coordination bonds from noncovalent interactions. In the latter, the distance between the electron rich and the spodium atoms is longer than the sum of the covalent radii but shorter than the sum of the van der Waals radii. In most Zn-dependent metalloenzymes, the spodium atom is bonded to three imidazole moieties belonging to the side chains of histidine amino-acids. Herein, in addition to the investigation of the SpB in the active site of three exemplifying enzymes, theoretical models where the Zn(II) atom is bonded either to three imidazole or triazole ligands are used in order to investigate the strength of the SpB and its competition with hydrogen bonding. A series of Lewis bases and anions have been used as SpB acceptors combined with six SpB donors (receptors) of general formula [ZnY3X]+ (Y = imidazole and triazole and X = Cl, N3 and SCH3). In addition to the investigation of the energetic and geometric features of the complexes, the SpB interactions have been further characterized using the natural bond orbital (NBO) method, quantum theory of “atoms-in-molecules” and the noncovalent interaction plot (NCI plot).


 Please wait while we load your content...
Please wait while we load your content...