Divide-and-link peptide docking: a fragment-based peptide docking protocol†
Abstract
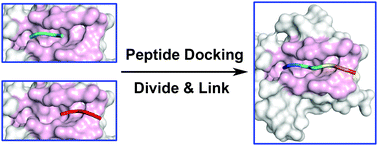
Protein–peptide interactions are crucial for various important cellular regulations, and are also a basis for understanding protein–protein interactions, protein folding and peptide drug design. Due to the limited structural data obtained using experimental methods, it is necessary to predict protein–peptide interaction modes using computational methods. In the present work, we designed a fragment-based docking protocol, Divide-and-Link Peptide Docking (DLPepDock), to predict protein–peptide binding modes. This protocol contains the following steps: dividing the peptide into fragments and separately docking the fragments using a third-party small molecular docking tool, linking the docked fragmental poses to form the whole peptide conformations via fragmental coordinate transformation using our in-house program, removing unreasonable poses according to several geometrical filters, extracting representative conformations after clustering for further minimization using the steepest descent and conjugation gradient methods based on a full-atom molecular force field and finally scoring using the MM/PBSA binding energy calculation implemented in Amber. When tested on the LEADS-PEP benchmark data set of 26 diverse complexes with peptides of 6–12 residues, FlexPepDock ab initio and AutoDock CrankPep achieved superior results. DLPepDock performed better than the other 15 docking protocols implemented in nine docking programs (HPepDock, DockThor, rDock, Glide, LeDock, AutoDock, AutoDock Vina, Surflex, and GOLD). The Linux scripts to call the third-party tools and run all the calculations.


 Please wait while we load your content...
Please wait while we load your content...