Improved carbon dioxide absorption in double-charged ionic liquids†
Abstract
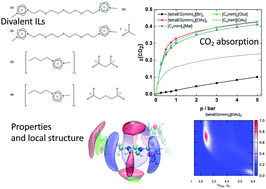
Four divalent ionic liquids based on imidazolium cations with alkyl or ether functionalized side-chains were synthesised and characterized: 3,3′-(tetraethyleneglycol-1,11-diyl)bis(1-methyl-1H-imidazolium)bromide, [tetraEG(mim)2][Br]2, 3,3′-(tetraethyleneglycol-1,11-diyl)bis(1-methyl-1H-imidazolium)acetate, [tetraEG(mim)2][OAc]2, 1-butyl-3-methylimidazolium malonate, [C4mim]2[Mal], and 3-butyl-1-methylimidazolium glutarate, [C4mim]2[Glut]. Their densities vary between 1.1 and 1.5 g cm−3 and their viscosities between 0.2 and 4 Pa s at 353 K. We found that the molar volumes are not additive, especially in the case of the divalent ionic liquids based on the double-charged imidazolium cations, meaning that they cannot be predicted using common group contribution methods. The reason for this behaviour could be explained by the structure of the cations, which is dominated by intramolecular hydrogen bonding. The carboxylate-based divalent ionic liquids absorb reversibly large quantities of carbon dioxide following a chemical mechanism described before. An improved 1 : 1 stoichiometry is achieved both in a double-charged imidazolium acetate ionic liquid and in imidazolium carboxylate salts with double charged anions. This behaviour places these ionic liquids amongst the best performing for carbon dioxide absorption.


 Please wait while we load your content...
Please wait while we load your content...