Determining chemical exchange rate constants in nanoemulsions using nuclear magnetic resonance†
Abstract
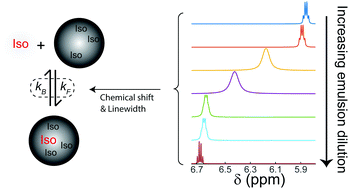
In this work, the second-order kinetics of molecules exchanging between chemically distinct microenvironments, such as those found in nanoemulsions, is studied using nuclear magnetic resonance (NMR). A unique aspect of NMR exchange studies in nanoemulsions is that the difference in molecular resonance frequencies between the two phases, which determines whether the exchange is fast, intermediate, or slow on the NMR timescale, can depend upon the emulsion droplet composition, which is also determined by the kinetic exchange constants themselves. Within the fast-exchange regime, changes in resonance frequencies and line widths with dilution were used to extract the exchange rate constants from the NMR spectra in a manner analogous to determining the kinetic parameters in NMR ligand binding experiments. As a demonstration, the kinetic exchange parameters of isoflurane release from an emulsification of isoflurane and perflurotributylamine (FC43) were determined using NMR dilution and diffusion studies.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...