The role of twisting in driving excited-state symmetry breaking and enhanced two-photon absorption in quadrupolar cationic pyridinium derivatives†
Abstract
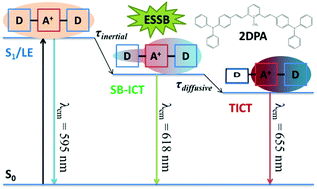
Two symmetric quadrupolar cationic push–pull compounds with a central electron-acceptor (N+-methylpyrydinium, A+) and different lateral electron-donors, (N,N-dimethylamino and N,N-diphenylamino, D) in a D–π–A+–π–D arrangement, were investigated together with their dipolar counterparts (D–π–A+) for their excited-state dynamics and NLO properties. As for the quadrupolar compounds, attention was focused on excited-state symmetry breaking (ESSB), which leads to a relaxed dipolar excited state. Both electron charge displacements and structural rearrangements were recognized in the excited-state dynamics of these molecules by resorting to femtosecond-resolved broadband fluorescence up-conversion experiments and advanced data analysis, used as a valuable alternative approach for fluorescent molecules compared to time-resolved IR spectroscopy, only suitable for compounds bearing IR markers. Specifically, intramolecular charge transfer (ICT) was found to be guided by ultrafast inertial solvation, while diffusive solvation can drive the twisting of lateral groups to originate twisted-ICT (TICT) states on a picosecond time scale. Yet still, only the bis-N,N-diphenylamino-substituted compound undergoes ESSB, in both highly and sparingly polar solvents, provided that it can experience large amplitude motions to a fully symmetry-broken TICT state. Besides well-known solvation effects, this structural requirement proved to be a necessary condition for these quadrupolar cations to undergo ESSB. In fact, a more efficient uncoupling between the out-of-plane D and A+ groups in the TICT state allows a greater stabilization gained through solvation, relative to the bis-N,N-dimethylamino-substituted derivative, which instead maintains its symmetry. This different behavior parallels the two-photon absorption (TPA) ability, which is greatly enhanced in the case of the bis-N,N-diphenylamino-substituted compound, paving the way for cutting-edge bio-imaging applications.


 Please wait while we load your content...
Please wait while we load your content...