Mechanistic origin of the time-dependence of the open-circuit voltage of a galvanic cell involving a ternary or higher compound
Abstract
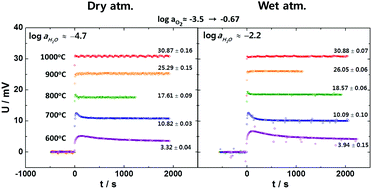
It has previously been predicted [H.-I. Yoo and M. Martin, Phys. Chem. Chem. Phys., 2010, 12, 14699] and observed [E. Kim, et al., Solid State Ionics, 2013, 235, 22] that the open-circuit voltage U of a galvanic cell, involving a ternary or higher compound with more than one kind of mobile ionic carrier, is path- and time-dependent upon imposition or removal of the mobile components’ chemical potential differences, in contradistinction to the cell involving a binary compound. This has been attributed [H.-I. Yoo and M. Martin, Phys. Chem. Chem. Phys., 2010, 12 14699; J.-Y. Yoon, et al., Solid State Ionics, 2012, 213, 22] to the decoupled redistributions of multiple mobile components or multi-fold relaxation. We hereby experimentally demonstrate with SrTi0.982Al0.018O3−Δ, known to have an appreciable water solubility depending on temperature, that introduction of a secondary ionic carrier H+ in addition to the native O2− indeed renders the otherwise time-independent U time-dependent; and that this phenomenon may, thus, be employed to probe the presence of a secondary ionic carrier, e.g., H+ in addition to the primary O2− in BaTi0.982Al0.018O3−Δ whose water solubility is yet to be known. The temporal behavior of U of SrTi0.982Al0.018O3−Δ subjected to the two fixed chemical potential differences, ΔμO and ΔμH, is precisely delineated in terms of two-fold relaxation of H and O, yielding their chemical diffusivity values, and consequently, the ambiguity with the EMF-method to determine the ionic transference numbers of a multinary compound is cleared away.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...