Dynamics of photodissociation of nitric oxide from S-nitrosylated cysteine and N-acetylated cysteine derivatives in water†
Abstract
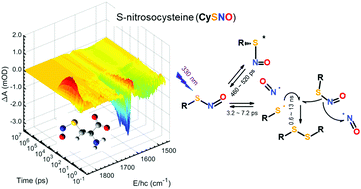
Cysteine and N-acetylated cysteine derivatives are ubiquitous in biological systems; they have thiol groups that bind NO to form S-nitrosothiols (RSNOs) such as S-nitrosocysteine (CySNO), S-nitroso-N-acetylcysteine (NacSNO), and S-nitroso-N-acetylpenicillamine (NapSNO). Although they have been utilised as thermally or catalytically decomposing NO donors, their photochemical applications are yet to be fully explored owing to the lack of photodissociation dynamics. To this end, the photoexcitation dynamics of these RSNOs in water at 330 nm were investigated using femtosecond time-resolved infrared (TRIR) spectroscopy over a broad time range encompassing the entire reaction, which includes the primary reaction, secondary reactions of the reaction intermediates, and product formation. We discovered that the acetate and amide groups in these RSNOs have strong vibrational bands sensitive to the bondage of NO and the electronic state of the compound, which facilitates the identification of reaction intermediates involved in photoexcitation. The simplest thiol available with the acetate group—thioglycolic acid—was nitrosylated; it produced S-nitrosothioglycolic acid (TgSNO) and was comparatively investigated. Transient absorption bands in the TRIR spectra of the RSNOs were assigned using quantum chemical calculations. Photoexcited cysteine-related RSNOs either decompose into RS and NO within 0.3 ps after excitation at 330 nm with a primary quantum yield (Φ1) of 0.46–1 or relax into an electronically excited intermediate state lying at 42 ± 3 kcal mol−1 above the ground state, which relaxes into the ground state with a time constant of 460–520 ps. A majority (62–80%) of the RS radical geminately rebinds with NO at a time constant of 3–7 ps. The remaining RS reacts with the neighbouring RSNO, which produces additional NO and RSSR with a (nearly) diffusion-limited rate constant that doubles the amount of NO produced; further, it remarkably extends the time window for the dissociated NO to react with the target compound. The final fraction of NO produced from these RSNOs at 330 nm was 0.32–0.58, and it depends on the geminate rebinding yield and Φ1. The detailed dynamics of the photoexcited RSNO can be utilised in the quantitative application of these RSNOs in practical use and in the synthesis of more efficient photoactivated NO precursors.
- This article is part of the themed collection: 2021 PCCP HOT Articles

 Please wait while we load your content...
Please wait while we load your content...