Molecular understanding of ion rejection in the freezing of aqueous solutions†
Abstract
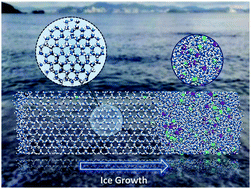
In this work, we investigate the microscopic mechanism of ion rejection phenomena during the freezing of aqueous NaCl solutions through molecular dynamics simulations. It is found that the hydration energy for the ion-water interaction is stronger than that between ions and ice, which is the fundamental reason giving rise to the phenomenon of ion rejection. The probability of ions being rejected by ice is determined by the competition between the energy barrier at the ice-water interface and the thermal effect. The ion rejection rate increases with increasing temperature. Furthermore, it is found that the rejection rate of Na+ is higher than that of Cl− because of the relatively large hydration energy difference between Na+–water and Na+–ice interactions. The role of temperature in the applications of ion rejection in freeze desalination is also discussed.


 Please wait while we load your content...
Please wait while we load your content...