Microscale pH inhomogeneity in frozen NaCl solutions†
Abstract
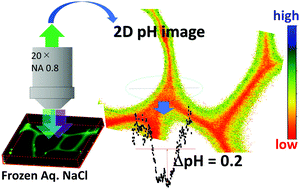
When an aqueous solution freezes at temperatures above the eutectic point, a freeze concentrated solution (FCS) is separated from the ice phase. Reactions of environmental importance often occur in the FCS and, in some cases, are accelerated compared to those in solution conditions. The pH of the FCS is an essential factor governing the thermodynamics and kinetics of the reactions occurring therein. It is known that freezing of aqueous NaCl causes an increase in the FCS pH, which arises from the difference in the partition to the ice phase between Na+ and Cl−. It has also been shown that H+ and other ions show surface-specific behaviors on ice. Although the details are not known, the ice/FCS interface can also affect the behaviors of ions. In this study, the pH distribution in the FCS is evaluated using ratiometric fluorescence microscopy, and the pH inhomogeneity is confirmed for frozen aqueous NaCl. However, interestingly, buffered solutions and frozen aqueous glycerol result in a uniform pH value. The pH in frozen NaCl is always higher near the ice/FCS interface than in the middle of the FCS vein.


 Please wait while we load your content...
Please wait while we load your content...