The dependence of the spectroscopic properties of orcein dyes on solvent proticity: insights from theory and experiments†
Abstract
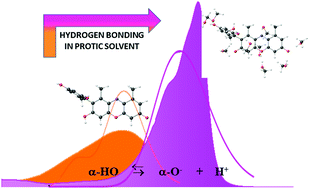
The electronic spectral properties of α-hydroxy-orcein (α-HO), one of the main components of the orcein dye, have been extensively investigated in solvents of different proticity through UV-Vis spectrophotometry combined with DFT and TDDFT calculations. The results highlight the occurrence of an acid–base equilibrium between the neutral (absorption maximum at 475 nm) and the monoanionic (absorption maximum at 578 nm) forms of the molecule. The position of this equilibrium was found to be sensitively dependent on solvent proticity, solution concentration and pH. Quantum mechanical calculations support the rationalization of the experimental data, confirming the key role of the protic solvent in shifting the acid–base equilibrium, through the establishment of hydrogen bond interactions on specific functional groups of the dye. Both deprotonation and dye coordination with protic solvent molecules determine the reduction of the HOMO–LUMO energy gap (0.71 eV), that can be related with the bathochromic effect envisaged both experimentally (0.59 eV) and theoretically (0.50 eV).


 Please wait while we load your content...
Please wait while we load your content...