Unraveling the stability of cyclobutadiene complexes using aromaticity markers†
Abstract
Cyclobutadiene (CBD) is the paradigmatic antiaromatic molecule but is known to form highly stable aromatic complexes, e.g. CBD–Fe(CO)3. This intriguing reversal of aromaticity from antiaromatic to aromatic terrain during the complexation process cannot be appropriately handled with single-reference-based theoretical techniques. We explore this aromaticity reversal, for the first time, by a detailed aromaticity analysis using magnetically induced current densities (MICD) and nucleus independent chemical shifts (NICS) using genuine ab initio multi-reference wavefunction-based theory. We trace the dramatic change of aromaticity for a prototypical cyclobutadiene complex, CBD–CH+ (CH+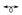 Fe(CO)3), considering a 3D potential energy surface for two independent parameters, namely the approach of CH+ and the automerization cross-section of cyclobutadiene. The 3D potential energy surfaces indicate the presence of a conical intersection/avoided crossing between the ground and the first excited state. The plot of aromaticity indices and the corresponding numerical values show that the change of aromaticity indices is drastic around the conical intersection/avoided crossing and automerization of cyclobutadiene plays a crucial role in the formation of cyclobutadiene complexes. Computations on analogous CBD–Be and CBD–CO systems (Be/CO
Fe(CO)3), considering a 3D potential energy surface for two independent parameters, namely the approach of CH+ and the automerization cross-section of cyclobutadiene. The 3D potential energy surfaces indicate the presence of a conical intersection/avoided crossing between the ground and the first excited state. The plot of aromaticity indices and the corresponding numerical values show that the change of aromaticity indices is drastic around the conical intersection/avoided crossing and automerization of cyclobutadiene plays a crucial role in the formation of cyclobutadiene complexes. Computations on analogous CBD–Be and CBD–CO systems (Be/CO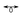 Fe(CO)3) emphasize the generality of the conclusions drawn from the CBD–CH+ system.
Fe(CO)3) emphasize the generality of the conclusions drawn from the CBD–CH+ system.



 Please wait while we load your content...
Please wait while we load your content...