Sequence-dependent aggregation-prone conformations of islet amyloid polypeptide†
Abstract
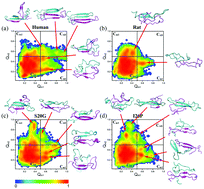
Amyloid proteins, which aggregate to form highly ordered structures, play a crucial role in various disease pathologies. Despite many previous studies on amyloid fibrils, which are an end product of protein aggregation, the structural characteristics of amyloid proteins in the early stage of aggregation and their related aggregation mechanism still remain elusive. The role of the amino acid sequence in the aggregation-prone structures of amyloid proteins at such a stage is not understood. Here, we have studied the sequence-dependent structural characteristics of islet amyloid polypeptide based on atomistic simulations and spectroscopic experiments. We show that the amino acid sequence determines non-bonded interactions that play a leading role in the formation of aggregation-prone conformations. Specifically, a single point mutation critically changes the population of aggregation-prone conformations, resulting in a change of the aggregation mechanism. Our simulation results were supported by experimental results suggesting that mutation affects the kinetics of aggregation and the structural characteristics of amyloid aggregates. Our study provides an insight into the role of sequence-dependent aggregation-prone conformations in the underlying mechanisms of amyloid aggregation.


 Please wait while we load your content...
Please wait while we load your content...