Theoretical study of the octahedral substitution effect in delaminated pyrophyllite: physicochemical properties and applications
Abstract
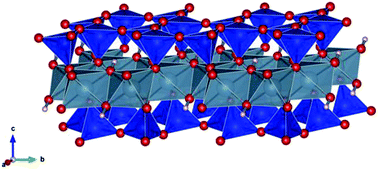
We have studied, using DFT calculations, some geometrical and electronic properties of delaminated pyrophyllite (D-P) and the corresponding layers that result from three isomorphic substitutions on octahedral sheets (Mg2+, Fe2+ and Fe3+). Bond lengths, layer thickness (dL), band gap (Eg), work function (WF), magnetic moment (μ), density of states and charge distributions are reported. These properties are important to control the behaviour of electronic devices. The results show that the layer thickness increases according to the ionic radius of the considered substituent. In the case of the three substitutions a reduction of the forbidden band is observed. Mg2+ induces a decrease in the Eg value of about 16.5% with respect to D-P, whereas for Fe this reduction is more significant due to the presence of trap states in the forbidden zone. For Fe2+ (Fe3+) the reduction in the Eg is around 62% (51%). Regarding the WF, our results showed that there is a decrease in its value after substitution. D-P has the highest WF value (8.15 eV), whereas the delaminated clay with Fe2+ has the lowest value (2.22 eV). Finally, D-P and D-P substituted with Mg2+ have a diamagnetic behaviour (μ = 0), whereas the presence of Fe2+ and Fe3+ induces a paramagnetic behaviour. The computed magnetic moment is 4 μB and 1 μB for D-P substituted with Fe2+ and Fe3+, respectively.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...