How does the solvent composition influence the transport properties of electrolyte solutions? LiPF6 and LiFSA in EC and DMC binary solvent†
Abstract
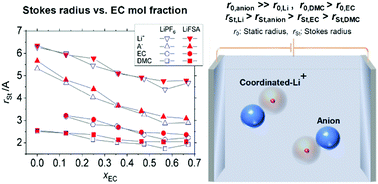
In this study, we experimentally measured the viscosity, η, and ionic conductivity, σ, of the electrolyte solutions of 1 mol kg−1 of LiPF6 or LiFSA dissolved in the binary mixture solvent of EC and DMC in a temperature range of 288 ≤ T/K ≤ 328 by varying the EC content from 0 to 60 vol%, which translates into the molar fraction of EC of 0 ≤ xEC ≤ 0.7. The diffusion coefficient, D, of each species, Li+, PF6−, FSA−, EC and DMC, was determined by pulse gradient spin-echo NMR. The state of molecules around Li+ was examined using the Raman spectra of the solvents and anions; the quantitative analysis suggests that EC is about twice as much preferred as DMC in the solvation shell at low xEC, while the EC-preference decreases with an increase in xEC. The classical Stokes–Einstein relation still quantitatively holds when evaluating the hydrodynamic radius, rSt, of transporting entities from D and η, in that (i) rSt,EC and rSt,DMC without the solute do not significantly differ from those in the solution; (ii) rSt,Li roughly coincides with the size estimated from the solvation number determined by Raman spectroscopy, which implies that rSt,Li reflects the solvation shell size; and (iii) rSt,anion is close to the static size, suggesting that anions are little solvated. The increase in xEC results in a decrease in rSt for all species, among which anions are most influenced, which is consistent with the view that the highly Li+-solvating EC, with its better dielectric shielding effect than DMC, liberates the anions from Li+, whereby enhancing the anion transfer that positively contributes to the ionic conductivity until the viscosity prevails at high xEC.

 Please wait while we load your content...
Please wait while we load your content...