First-principles investigation of CO and CO2 adsorption on pristine and Fe-doped planar carbon allotrope net-Y
Abstract
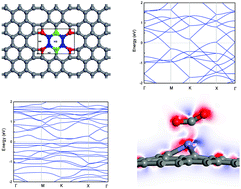
A new planar carbon allotrope named net-Y with periodic four-six-eight-membered carbon rings has been synthesized in this study. In this paper, the adsorption properties of CO and CO2 on the pristine net-Y and two Fe-doped net-Y surfaces were studied using first-principles calculations. Using adsorption energy, charge analysis, adsorption distance, deformation charge density and density of states, it can be found that the adsorption of CO and CO2 on the pristine net-Y surface appears as physisorption with weak interactions. Based on this, in order to enhance the adsorption strength of CO and CO2 on the surface of net-Y, Fe-doping is performed on net-Y. The calculation results after doping show that the doping of Fe atoms at different positions can significantly improve the adsorption strength of the two gas molecules. After CO and CO2 are adsorbed on the doped net-Y surface, the adsorption energy is greatly improved compared with the pristine adsorption energy, which makes the two gas molecules form a chemical bond with the substrate. Subsequently, the results of charge density and density of states further prove that the adsorption system shows chemisorption with strong interactions. Comparing doped and pristine adsorption, after Fe doping, the adsorption of CO and CO2 is stronger, which makes it possible to use this material effectively for the adsorption and removal of CO and CO2 gases.


 Please wait while we load your content...
Please wait while we load your content...