A nonlinear evolution of the structure, morphology, and optical properties of PbS–CdS films with cadmium nitrate in the reaction mixture
Abstract
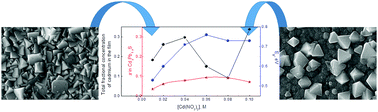
Herein, we describe the nonlinear processes for the formation of thin films of the PbS–CdS system using chemical bath deposition with a gradual change in the cadmium nitrate content in the reaction mixture. The morphology of films was studied via scanning electron microscopy and atomic force microscopy. The mechanism for the formation of thin-film compounds can be considered as cluster-particle aggregation (diffusion-limited aggregation). X-ray diffraction confirmed the formation of single-phase layers of substitutional B1-type CdxPb1−xS solid solutions (space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) when the concentration of cadmium nitrate in the reaction bath increased up to [Cd(NO3)2] = 0.08 M. The maximum cadmium content in CdxPb1−xS solid solutions was determined to be x = 0.094. At the concentration of [Cd(NO3)2] = 0.10 M, a two-phase film was formed, where the film consisted of crystallites of cubic CdxPb1−xS with x = 0.071 (lower compared to the film obtained at [Cd(NO3)2] = 0.08 M) and fine-crystalline hexagonal B4-type Cd1−δS (space group P63mc). The texture of grains forming films was observed; where a predominant orientation with the (111) plane along substrate in PbS changed to the (200) plane in CdxPb1−xS films, the portion of (200) oriented grains increased with an increase in cadmium nitrate content up to [Cd(NO3)2] = 0.08 M, and at 0.10 M of cadmium nitrate, a radical change in the type of texture to the (111) type occurred. The concentration [Cd(NO3)2] = 0.10 M is called the critical concentration, where under this condition, the deposition process occurs due to the excess Gibbs energy. The higher cadmium content x in films determined by the energy-dispersive X-ray analysis and Auger spectroscopy compared with that estimated from the crystal lattice parameter is associated with the presence of an additional amorphous CdS phase formed as a sublayer distributed in intercrystalline spaces and island formations. Optical studies showed a nonlinear change in the band gap (Eg) of obtained films from 0.53 to 0.76 eV, whereas at the critical cadmium salt concentration (0.10 M), two crystalline phases with Eg equal to 0.73 and 2.47 eV were observed.
m) when the concentration of cadmium nitrate in the reaction bath increased up to [Cd(NO3)2] = 0.08 M. The maximum cadmium content in CdxPb1−xS solid solutions was determined to be x = 0.094. At the concentration of [Cd(NO3)2] = 0.10 M, a two-phase film was formed, where the film consisted of crystallites of cubic CdxPb1−xS with x = 0.071 (lower compared to the film obtained at [Cd(NO3)2] = 0.08 M) and fine-crystalline hexagonal B4-type Cd1−δS (space group P63mc). The texture of grains forming films was observed; where a predominant orientation with the (111) plane along substrate in PbS changed to the (200) plane in CdxPb1−xS films, the portion of (200) oriented grains increased with an increase in cadmium nitrate content up to [Cd(NO3)2] = 0.08 M, and at 0.10 M of cadmium nitrate, a radical change in the type of texture to the (111) type occurred. The concentration [Cd(NO3)2] = 0.10 M is called the critical concentration, where under this condition, the deposition process occurs due to the excess Gibbs energy. The higher cadmium content x in films determined by the energy-dispersive X-ray analysis and Auger spectroscopy compared with that estimated from the crystal lattice parameter is associated with the presence of an additional amorphous CdS phase formed as a sublayer distributed in intercrystalline spaces and island formations. Optical studies showed a nonlinear change in the band gap (Eg) of obtained films from 0.53 to 0.76 eV, whereas at the critical cadmium salt concentration (0.10 M), two crystalline phases with Eg equal to 0.73 and 2.47 eV were observed.


 Please wait while we load your content...
Please wait while we load your content...