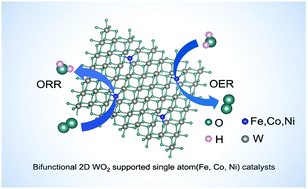
Bifunctional electrocatalysts for oxygen reduction and oxygen evolution: a theoretical study on 2D metallic WO2-supported single atom (Fe, Co, or Ni) catalysts†
Abstract
Catalysts play a critical role in the oxygen evolution reaction (OER) and the oxygen reduction reaction (ORR) for energy storage, conversion, and utilization. Herein, first-principles density functional theory (DFT) calculations demonstrated that single-metal-atom (Fe, Co, or Ni) sites can bind to the surface of 2D WO2, enhancing the adsorption of intermediates involved in the OER/ORR. Furthermore, it was found that the single-metal-atom-doped 2D WO2 achieves the smallest OER and ORR overpotentials of 0.42 V and 0.40 V, respectively, which are comparable to those of IrO2 or Pt-based catalysts. This predicts the excellent OER/ORR catalytic activities of the single-metal-atom (Fe, Co, or Ni) doped 2D WO2, which would be a promising bifunctional catalyst for fuel cells, water splitting, and metal–air batteries.

 Please wait while we load your content...
Please wait while we load your content...