Energy density of high-pressure nitrogen-rich MNx compounds†
Abstract
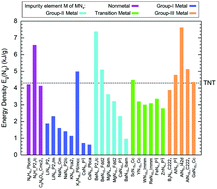
In the past decade, a large number of nitrogen-rich MNx compounds have been discovered under high-pressure conditions. In this work, we have evaluated the energy densities of MNx structures with thermodynamic and dynamical stability through first-principles calculations. The results show that the energy densities of MNx consisting of alkali metals and cyclo-N5− are less than ∼0.5 TNT equivalence, whereas the group-III metal nitrides have high-energy density regardless of the type of nitrogen oligomers in the structures. To clarify the energy density difference for MNx composed of different impurities, bivariate Pearson correlation analysis is performed, which reveals that the high-energy density of MNx is related to the large N density, small M atomic radius, short M–N bond length, small MNx ionicity, long N–N bond length and large M formal oxidation state. According to this correlation, H, Be, B, Al, Si and P elements have been proposed as the candidate impurities to synthesize high-energy density MNx.


 Please wait while we load your content...
Please wait while we load your content...