From absolute potentials to a generalized computational standard hydrogen electrode for aqueous and non-aqueous solvents†
Abstract
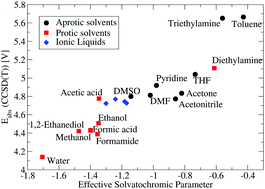
We describe a simple and efficient procedure to compute a conversion factor for the absolute potential of the standard hydrogen electrode in water to any other solvent. In contrast to earlier methods our procedure only requires the pKa of an arbitrary acid in water and few simple quantum chemical calculations as input. Thus, it is not affected adversely by experimental shortcomings related to measurements in non-aqueous solvents. By combining this conversion factor with the absolute potential in water, the absolute potential in the solvent of interest is obtained. Based on this procedure a new generalized computational standard hydrogen electrode for the computation of electron transfer and proton-coupled electron transfer potentials in non-aqueous solvents and ionic liquids is developed. This enables for the first time the reliable prediction of redox potentials in any solvent. The method is tested through calculation of absolute potentials in 36 solvents. Using the Kamlet–Taft linear solvation energy model we find that the relative absolute potentials consistently increase with decreasing polarisability and decreasing hydrogen bonding ability. For protic solvents good agreement with literature is observed while significant deviations are found for aprotic solvents. The obtained conversion factors are independent of the quantum chemical method, while minor differences are observed between solvation models. This does, however, not affect the global trends.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...