Effects of support and oxygen vacancies on the energetics of NiO reduction with H2 for the chemical looping combustion (CLC) reaction; a DFT study†
Abstract
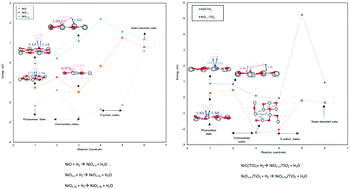
Chemical looping combustion (CLC) technology is an innovative energy conversion technology that employs oxygen carriers (OC), typically metal oxides, to burn fossil fuels with a minimal carbon footprint. The performance of OCs can be enhanced by the support on which they are deposited through two mechanisms acting at different scales, viz., microstructural and synergetic effects. In this work, the synergetic effect of NiO supported on TiO2 in reaction with hydrogen as a fuel is studied using density functional theory (DFT). Changes in the energetics of the NiO-hydrogen reaction are explained as a consequence of the interaction between the TiO2 support and NiO. The results indicate that the electronic interaction of the TiO2 support with NiO lowers the energy of intermediate states and the energy of the reaction. The effect of TiO2 increases with the creation of more O vacancies as the reaction proceeded. This enhanced reactivity of the NiO-hydrogen reaction is attributed to both an electronic effect of TiO2 and a geometric effect due to O vacancy creation. The synergetic effect of the support on the OC reactions at the atomic level reported here can pave the path to differentiate the electronic and geometric effects and establish the knowledge for the rational design of OC and support systems.


 Please wait while we load your content...
Please wait while we load your content...