Bonding of C1 fragments on metal nanoclusters: a search for methane conversion catalysts with swarm intelligence†
Abstract
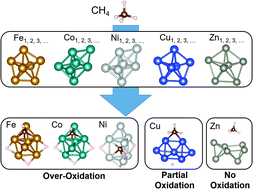
There is a need for a catalyst that can directly convert methane into useful substances. The use of Ni as a catalyst for the steam reforming of methane has led us to look at Ni nanoclusters as potential candidates for the direct conversion of methane. Fe, Co, Cu, and Zn nanoclusters are also focused on. How the type of C1 fragments (CH4, CH3, CH2, CH, and C) stabilized by the metal nanoclusters as adsorbed species varies with metal species is theoretically investigated. The particle swarm optimization algorithm, which is based on swarm intelligence, as well as density functional theory, is used for this calculation. The Ni nanoclusters are found to preferentially adsorb C as a stable species, the Fe and the Co nanoclusters both CH and CH3, and the Cu nanoclusters CH3; the Zn nanoclusters are found not to chemisorb any C1 fragment. The methane activation capacity can be ranked in the order of Ni > Fe > Co > Cu > Zn. The highest methane activation capacity of Ni is due to the strongest covalent nature of the interaction between Ni and the adsorbed species. The ionicity of the bond between Fe and the adsorbed species is higher than that between Co and the adsorbed species, while the covalent nature of the bonds is comparable for both. The weak methane activation ability of Cu compared to Fe, Co, and Ni is found to be due to the fact that both the covalent and ionic bond strengths between Cu and the adsorbed species are weak. Zn and the adsorbed species form neither ionic nor covalent bonds. These results indicate that the Fe and the Co nanoclusters as well as the Ni may lead to the over-oxidation of methane, whereas the Zn nanoclusters cannot activate methane in the first place; therefore, their application to direct methane conversion catalysts is unlikely. Since the Cu nanoclusters do not adsorb C and CH as stable species, but CH3 stably, the Cu nanoclusters are expected to work as a catalyst for the direct conversion of methane.


 Please wait while we load your content...
Please wait while we load your content...