Observation of pressure-induced electron transfer in SnC2O4
Abstract
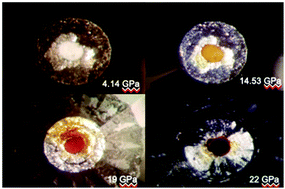
We examined the high pressure behavior of stannous oxalate via Raman and X-ray absorption spectroscopy (XAS) inside a diamond anvil cell. Phase transitions were observed to occur near 2.6 and 15 GPa which were reversible upon decompression to ambient conditions. When further pressurized above 15 GPa, the colorless material sustains irreversible chemical alterations and becomes bright red colored – darkening at higher pressures. Another irreversible phase transition occurred above 20 GPa. Concomitant with color change of the sample, we observed a softening of the ν(C–C) modes of the C2O42− anion via Raman spectroscopy. We performed a separate XAS experiment which indicates that the Sn2+ cation undergoes a partial reduction of the 2+ oxidation state with pressure which persists when the sample was depressurized to ambient conditions. Thus, electron density within the C–C bond in the oxalate anion appears to migrate toward the tin cation with pressure. This observation suggests that pressure can offer a very controllable means to vary cation–anion and unit cell dimensions (and thus the electric interactions causing electron movement) and thus the pressure-induced synthesis of novel materials.


 Please wait while we load your content...
Please wait while we load your content...