Ab initio dynamics simulation of laser-induced photodissociation of phenol†
Abstract
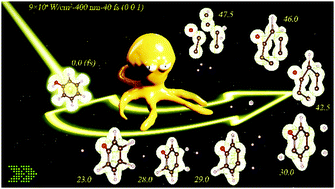
We theoretically investigated the photodissociation dynamics of phenol molecules steered by a sequence of temporally shaped femtosecond laser pulses with high intensity and ultrashort duration, via the real-time Time-Dependent Density Functional Theory (rt-TDDFT) combined with a Molecular Dynamics (MD) simulation. The principal findings of this research are that the phenol photodissociation can take place in 50 fs; the bonds broke sequentially; the degree of phenol molecular dissociation has a strong linear correlation with the intensity. For an incident laser being 800 nm–40 fs (wavelength-pulse duration), the threshold intensity is 7 × 1014 W cm−2 and the products are hydrogen from OH1 (phenolic hydroxyl group) and C6H5O-fragments. More fragments will be found at stronger intensity, shorter wavelengths, and longer pulse duration. More accurately, we estimated the critical values of bond cleavage of an isolated phenol molecule are 1.779 Å for O–H1 and 2.184 Å for C–Hs via Electron localization function (ELF) analysis. The photodissociation of the phenol molecule was triggered via the excitation of electrons and the dissociation process of phenol here is in good agreement with the characteristics of field-assisted dissociation (FAD) theory. Orthogonal tests with an L9 (34) matrix and threshold intensity decrease tests were conducted to confirm the mechanism. Our research gives an insight into the photodissociation experiment of phenol and provides a simple yet effective way to understand the photochemical experiments of more complex organic pollutants with toxicity.


 Please wait while we load your content...
Please wait while we load your content...