Cyclopentadienyl radical formation from the reaction of excited nitrogen atoms with benzene: a theoretical study†
Abstract
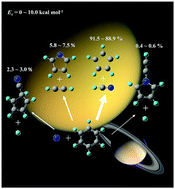
Ab initio CCSD(T)/CBS//ωB97X-D/6-311+G(d,p) calculations of the C6H6N potential energy surface were performed to investigate the reaction mechanism underlying the reaction of atomic nitrogen (2D) with benzene. Thereafter, Rice–Ramsperger–Kassel–Marcus (RRKM) calculations of reaction rate constants and product branching ratios were performed under single-collision conditions. The results revealed that the N(2D) + C6H6 reaction in the case of statistical behavior is expected to produce hydrogen cyanide plus a cyclopentadienyl radical (91.5–88.9%), acetylene plus a pyrrole radical (5.8–7.5%), 1-cyano-2,4-cyclopentadiene + H (2.3–3.0%) and 1-ethynyl-pyrrole + H (0.4–0.6%), with the most favorable pathways being the initial adduct i1 leading to the formation of a seven-membered cyclic intermediate i12 through an exothermic ring expansion process and a multistep route i12 → i15 → i16 → C5H5 + HCN featuring an intramolecular ring-shrinking process involving a C–C bond fusion elimination channel to yield the bicyclic intermediate i15, followed by hydrogen cyanide elimination, thus forming a cyclopentadienyl radical. The calculated product branching ratios were consistent with the available experimental data; however, some quantitative deviations from the experimental results and the possible reasons are also discussed. The possible effects of the title reaction on the upper atmosphere of Titan, with critical implications for the rapid degradation of nitrogen-bearing polycyclic aromatic hydrocarbons, were compared with the mass growth processes of their polycyclic aromatic hydrocarbon counterparts produced through ring expansion.


 Please wait while we load your content...
Please wait while we load your content...