A theoretical study on the excited-state deactivation paths for the A–5FU dimer†
Abstract
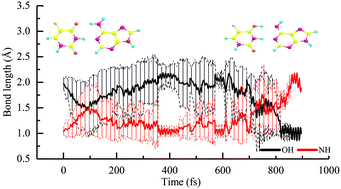
The photostability of DNA plays a key role in the normal function of organisms. A–5FU is a base pair derivative of the A–T dimer where the methyl group is replaced by a F atom. Here, accurate static TDDFT calculations and non-adiabatic dynamic simulations are used to systematically investigate the excited-state decay paths of the A–5FU dimer related to the proton transfer and the out-of-plane twisting deformation motion of A and 5FU in the 1ππ* and 1nπ* states. CC2 is used to check the accuracy of the current TDDFT calculations. Our results show that the deformation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C or C
C or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond in A and 5FU provides an efficient pathway for the depopulation of the lowest excited states, which can compete with the excited-state proton transfer paths in the dimer. This finding indicates that monomer-like decay paths could be important for the photostability of weakly hydrogen-bonded DNA base pairs and provide a new insight into the excited-state decay paths in base pairs and their analogues.
N double bond in A and 5FU provides an efficient pathway for the depopulation of the lowest excited states, which can compete with the excited-state proton transfer paths in the dimer. This finding indicates that monomer-like decay paths could be important for the photostability of weakly hydrogen-bonded DNA base pairs and provide a new insight into the excited-state decay paths in base pairs and their analogues.


 Please wait while we load your content...
Please wait while we load your content...