Liquid droplets of protein LAF1 provide a vehicle to regulate storage of the signaling protein K-Ras4B and its transport to the lipid membrane†
Abstract
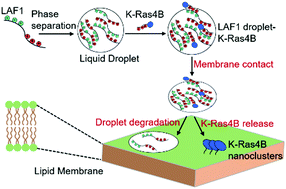
Liquid–liquid phase separation has been shown to promote the formation of functional membraneless organelles involved in various cellular processes, including metabolism, stress response and signal transduction. Protein LAF1 found in P-granules phase separates into liquid-like droplets by patterned electrostatic interactions between acidic and basic tracts in LAF1 and has been used as model system in this study. We show that signaling proteins, such as K-Ras4B, a small GTPase that acts as a molecular switch and regulates many cellular processes including proliferation, apoptosis and cell growth, can colocalize in LAF1 droplets. Colocalization is facilitated by electrostatic interactions between the positively charged polybasic domain of K-Ras4B and the negatively charged motifs of LAF1. The interaction partners B- and C-Raf of K-Ras4B can also be recruited to the liquid droplets. Upon contact with an anionic lipid bilayer membrane, the liquid droplets dissolve and K-Ras4B is released, forming nanoclusters in the lipid membrane. Considering the high tuneability of liquid–liquid phase separation in the cell, the colocalization of signaling proteins and their effector molecules in liquid droplets may provide an additional vehicle for regulating storage and transport of membrane-associated signaling proteins such as K-Ras4B and offer an alternative strategy for high-fidelity signal output.


 Please wait while we load your content...
Please wait while we load your content...