Theoretical insights on the excited-state-deactivation mechanisms of protonated thymine and cytosine†
Abstract
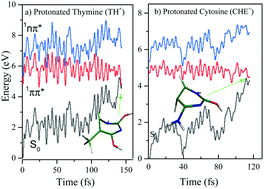
Ab initio and surface-hopping nonadiabatic dynamics simulation methods were employed to investigate relaxation mechanisms in protonated thymine (TH+) and cytosine (CH+). A few conical intersections were located between 1ππ* and S0 states for each system with the CASSCF (8,8) theoretical model and relevant contributions to the deactivation mechanism of titled systems were addressed by the determination of potential energy profiles at the CASPT2 (12,10) theoretical level. It was revealed that the relaxation of the 1ππ* state of the most stable conformer of both systems to the ground state is mostly governed by the accessible S1/S0 conical intersection resulting from the barrier-free out-of-plane deformation. Interestingly, it was exhibited that the ring puckering coordinate driven from the C6 position of the heterocycle ring in TH+ and CH+ plays the most prominent role in the deactivation mechanism of considered systems. Our ab initio results are also supported by excited-state nonadiabatic dynamics simulations based on ADC(2), describing the ultrashort S1 lifetime of TH+/CH+ by analyzing trajectories leading excited systems to the ground. It was confirmed that the excited-state population mostly relaxes to the ground via the ring puckering coordinate from the C6 moiety. Overall, the theoretical results of this study shed light on the deactivation mechanism of protonated DNA bases.


 Please wait while we load your content...
Please wait while we load your content...