First-principles screening of single transition metal atoms anchored on two-dimensional C9N4 for the nitrogen reduction reaction†
Abstract
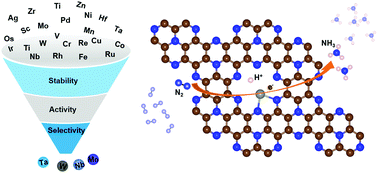
Compared to the Haber–Bosch process, the electrochemical nitrogen reduction reaction (NRR) can convert N2 into NH3 under ambient conditions, and thus has attracted considerable attention in recent years. However, it remains a challenge to fabricate NRR catalysts with high faradaic efficiency and yield rate. In this work, by systematic first-principles calculations, we investigate the structure, stability and catalytic performance of single metal atoms anchored on porous monolayer C9N4 (M@C9N4) for the electrochemical NRR. A total of 25 transition metals (Sc–Zn, Zr–Mo, Ru–Ag, Hf–Au) were explored, and we screened out four promising systems, i.e., Nb, Ta, Re and W@C9N4, which not only exhibit high catalytic activity with low limiting potentials of −0.3, −0.42, −0.49 and −0.25 V, respectively, but also have superior selectivity that suppresses the competitive hydrogen evolution reaction. The physical origin lies in the coupling between the d orbitals of the transition metals and the 2π* orbital of N2, which activates the N2 molecule and facilitates the reduction process. Our proposed systems are kinetically and thermodynamically stable, which may shed light on future design and fabrication of high-efficiency single atom catalysts for various technologically important chemical reactions.


 Please wait while we load your content...
Please wait while we load your content...