Structural, elastic and electronic properties of atomically thin pyridyne: theoretical predictions†
Abstract
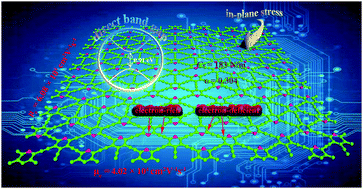
In this paper, we propose a new acetylenic carbon material called pyridyne, which is composed of acetylenic linkages and pyridine rings. From first-principles calculations, we investigate the structural, elastic and electronic properties of pyridyne. It is found that the structure of pyridyne is stable at 300 K and its stability is comparable to experimentally synthesized graphdiyne and graphtetrayne. Compared with graphene or graphyne, pyridyne possesses more diverse pores and reduced delocalization of electrons. The in-plane stiffness of pyridyne is 183 N m−1 with a Poisson's ratio of 0.304. Pyridyne is found to be a semiconductor with a direct band gap of 0.91 eV. The intrinsic electron mobility can reach 6.08 × 104 cm2 V−1 s−1, while the hole mobility can reach 1.82 × 104 cm2 V−1 s−1.


 Please wait while we load your content...
Please wait while we load your content...