Dissolution kinetics of a sodium borosilicate glass in Tris buffer solutions: impact of Tris concentration and acid (HCl/HNO3) identity†
Abstract
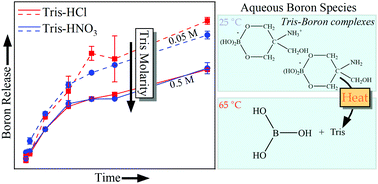
Understanding the corrosion behavior of glasses in near-neutral environments is crucial for many technologies including glasses for regenerative medicine and nuclear waste immobilization. To maintain consistent pH values throughout experiments in the pH = 7 to 9 regime, buffer solutions containing tris(hydroxymethyl)aminomethane (“Tris”, or sometimes called THAM) are recommended in ISO standards 10993-14 and 23317 for evaluating biomaterial degradation and utilized throughout glass dissolution behavior literature—a key advantage being the absence of dissolved alkali/alkaline earth cations (i.e. Na+ or Ca2+) that can convolute experimental results due to solution feedback effects. Although Tris is effective at maintaining the solution pH, it has presented concerns due to the adverse artificial effects it produces while studying glass corrosion, especially in borosilicate glasses. Therefore, many open questions still remain on the topic of borosilicate glass interaction with Tris-based solutions. We have approached this topic by studying the dissolution behavior of a sodium borosilicate glass in a wide range of Tris-based solutions at 65 °C with varied acid identity (Tris–HCl vs. Tris–HNO3), buffer concentration (0.01 M to 0.5 M), and pH (7–9). The results have been discussed in reference to previous studies on this topic and the following conclusions have been made: (i) acid identity in Tris-based solutions does not exhibit a significant impact on the dissolution behavior of borosilicate glasses, (ii) ∼0.1 M Tris-based solutions are ideal for maintaining solution pH in the absence of obvious undesirable solution chemistry effects, and (iii) Tris–boron complexes can form in solution as a result of glass dissolution processes. The complex formation, however, exhibits a distinct temperature-dependence, and requires further study to uncover the precise mechanisms by which Tris-based solutions impact borosilicate glass dissolution behavior.


 Please wait while we load your content...
Please wait while we load your content...