Third dissociation constant of phosphoric acid in H2O and D2O from 75 to 300 °C at p = 20.4 MPa using Raman spectroscopy and a titanium-sapphire flow cell†
Abstract
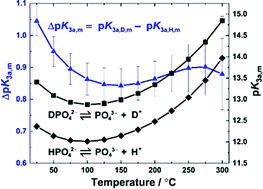
A custom-built titanium-sapphire flow cell has been used with a confocal Raman microscope to collect solvent-corrected reduced isotropic spectra of sodium and potassium phosphate solutions in light and heavy water from 75 to 300 °C at 20.4 ± 0.4 MPa over a wide range of concentrations. The symmetric vibrational modes of PO43− and HPO42−/DPO42− in both solvents broadened and moved to lower wavenumbers with increasing temperature, suggesting that oxyanion–water hydrogen bond strengths increase at elevated temperatures. Raman scattering coefficients, measured relative to the trifluoromethanesulfonate ion, were used to determine thermodynamic equilibrium quotients for the reaction PO43− + H2O ⇌ HPO42− + OH− and its deuterium counterpart. Standard-state acid ionization constants were calculated using a modified Pitzer model and fitted as a function of temperature and solvent molar volume over the range of 25 to 300 °C from psat to 20 MPa. The deuterium isotope effect on the chemical equilibrium constant, ΔpK3a,m = pK3a,D,m − pK3a,H,m, was found to decrease from 1.045 ± 0.046 at 25 °C to 0.898 ± 0.073 at 250 °C. This behaviour is consistent with a model in which zero-point energy effects dominate at low temperatures and long-range solvent polarization becomes increasingly important as the temperature increases towards the critical point of D2O. These are the first experimental ionization constants to be reported in the literature for this reaction in light water above 50 °C and in heavy water at any temperature.


 Please wait while we load your content...
Please wait while we load your content...