Functionalised nanoclays as microstructure modifiers for calcium and magnesium silicate hydrates†
Abstract
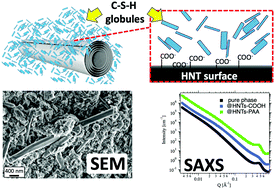
Calcium silicate hydrate (C–S–H) is the main binding product of ordinary Portland concrete (OPC). Unfortunately, OPC production generates ∼5% of all anthropomorphic CO2. Among the most promising green alternatives, magnesium silicate hydrate (M–S–H) is a colloidal gel equivalent to C–S–H which exhibits weaker mechanical properties. Here we investigated the effect of the inclusion of aluminosilicate nanoclays (HNTs) on the microstructure of the silicate hydrate gels as a strategy to ultimately improve their mechanical properties. The microstructure of C–S–H and M–S–H gels synthesized with and without carboxylic or polycarboxylic functionalised HNTs (HNT–COOH, HNT–PAA) was investigated by a multi-technique approach including small- and wide-angle X-ray scattering (SWAXS) and scanning electron microscopy (SEM). The results indicate that, during C–S–H formation in solution, HNTs decrease the size of the disk-like globules with little influence on the spacing of calcium silicate layers. In the case of M–S–H, the presence of functionalised HNTs has a reduced effect on the hydrate structure as a result of the weaker interaction of the carboxylic moieties with Mg2+ ions. SEM investigation on the synthesized composites shows that HNT–PAA are better included in the hydration products. Moreover, in the proximity of the PAA functionalised surfaces, less extended aggregates are formed. The morphology at the micron scale for M–S–H and C–S–H with HNT–COOH is conserved.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...