Experimental and computational diagnosis of the fluxional nature of a benzene-1,3,5-tricarboxamide-based hydrogen-bonded dimer†
Abstract
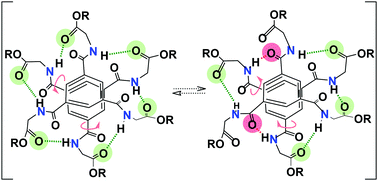
Precise characterization of the hydrogen bond network present in discrete self-assemblies of benzene-1,3,5-tricarboxamide monomers derived from amino-esters (ester BTAs) is crucial for the construction of elaborated functional co-assemblies. For all ester BTA dimeric structures previously reported, ester carbonyls in the side chain acted as hydrogen bond acceptors, yielding well-defined dimers stabilized by six hydrogen bonds. The ester BTA monomer derived from glycine (BTA Gly) shows a markedly different self-assembly behaviour. We report herein a combined experimental and computational investigation aimed at determining the nature of the dimeric species formed by BTA Gly. Two distinct dimeric structures were characterized by single-crystal X-ray diffraction measurements. Likewise, a range of spectroscopic and scattering techniques as well as molecular modelling were employed to diagnose the nature of dynamic dimeric structures in toluene. Our results unambiguously establish that both ester and amide carbonyls are involved in the hydrogen bond network of the discrete dimeric species formed by BTA Gly. The participation of roughly 4.5 ester carbonyls and 1.5 amide carbonyls per dimer as determined by FT-IR spectroscopy implies that several conformations coexist in solution. Moreover, NMR analysis and modelling data reveal rapid interconversion between these different conformers leading to a symmetric structure on the NMR timescale. Rapid hydrogen bond shuffling between conformers having three (three), two (four), one (five) and zero (six) amide carbonyl groups (ester carbonyl groups, respectively) as hydrogen bond acceptors is proposed to explain the magnetic equivalence of the amide N–H on the NMR timescale. When compared to other ester BTA derivatives in which only ester carbonyls act as hydrogen bond acceptors, the fluxional behaviour of the hydrogen-bonded dimers of BTA Gly likely originates from a larger range of energetically favorable conformations accessible through rotation of the BTA side chains.


 Please wait while we load your content...
Please wait while we load your content...