The effect of nanoparticulate PdO co-catalysts on the faradaic and light conversion efficiency of WO3 photoanodes for water oxidation†
Abstract
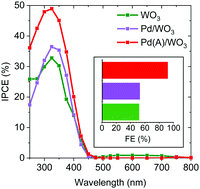
WO3 photoanodes offer rare stability in acidic media, but are limited by their selectivity for oxygen evolution over parasitic side reactions, when employed in photoelectrochemical (PEC) water splitting. Herein, this is remedied via the modification of nanostructured WO3 photoanodes with surface decorated PdO as an oxygen evolution co-catalyst (OEC). The photoanodes and co-catalyst particles are grown using an up-scalable aerosol assisted chemical vapour deposition (AA-CVD) route, and their physical properties characterised by X-ray diffraction (XRD), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HR-TEM) and UV-vis absorption spectroscopy. Subsequent PEC and transient photocurrent (TPC) measurements showed that the use of a PdO co-catalyst dramatically increases the faradaic efficiency (FE) of water oxidation from 52% to 92%, whilst simultaneously enhancing the photocurrent generation and charge extraction rate. The Pd oxidation state was found to be critical in achieving these notable improvements to the photoanode performance, which is primarily attributed to the higher selectivity towards oxygen evolution when PdO is used as an OEC and the formation of a favourable junction between WO3 and PdO, that drives band bending and charge separation.


 Please wait while we load your content...
Please wait while we load your content...