A vibrational spectroscopic and computational study of the structures of protonated imidacloprid and its fragmentation products in the gas phase†
Abstract
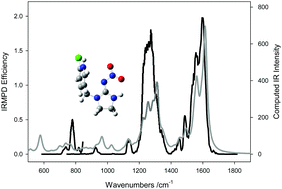
Infrared multiple photon dissociation (IRMPD) spectroscopy experiments in the 600–2000 cm−1 region and computational chemistry studies were combined with the aim of elucidating the structures of protonated imidacloprid (pIMI), and its unimolecular decomposition products. The computed IR spectra for the lowest energy structures for pIMI as well as for protonated desnitrosoimidacloprid, corresponding to the loss of NO radical (pIMI-NO), and protonated imidacloprid urea corresponding to the loss of N2O (pIMIU) were found to reproduce the experimental IRMPD spectrum quite well. The complex IRMPD spectrum for protonated desnitroimidaclpride (pDIMI), resulting from the loss of NO2 radical from pIMI, was explained as a contribution from several computed structures, including those involving simple loss of NO2 radical and some isomerization. However, based on a comparison of the computed IR spectrum for the lowest energy structure of pDIMI and the IRMPD spectrum, it was concluded that the lowest energy structure is a minor contributor to the experimental spectrum. This observation is rationalized as being due to the energy requirement for isomerization to the lowest energy structure, being substantially higher than that for simple loss of NO2 radical. Experimental mass spectrometry fragmentation results indicated that the loss of N, O2, H was the result of a loss of NO radical followed by loss of OH radical. A comparison of the experimental IRMPD and computed IR spectra revealed that following NO radical loss, the structure entailing a hydride shift from the methylene bridge to the guanidine moiety followed by OH radical elimination, generated the best match with the experimental IRMPD spectrum. This was consistent with the computed potential energy surfaces showing this structure as having the lowest energy requirement.


 Please wait while we load your content...
Please wait while we load your content...