Conformational sampling and large amplitude motion of methyl valerate†
Abstract
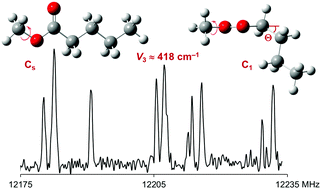
The microwave spectrum of the fruit ester methyl valerate was recorded using two molecular jet Fourier transform spectrometers covering the frequency range from 2 to 40 GHz. Quantum chemical calculations yielded 11 minima for the anti ester configuration, among them two were identified in the experimental spectrum. The methyl group in the methoxy moiety undergoes internal rotation, leading to torsional splittings of all rotational transitions into doublets. The barrier to internal rotation of the methoxy methyl group was deduced to be 417.724(70) cm−1 and 418.059(27) cm−1 for the C1 and the Cs conformer, respectively, essentially the same values as those in methyl alkanoates with shorter alkyl chains, which are methyl acetate, methyl propionate and methyl butyrate. Geometry parameters such as the rotational and centrifugal distortion constants could be determined with very high accuracy. Optimisations at different levels of theory were performed for a comparison with the experimental results. The MP2/6-311++G(d,p) level of theory failed to calculate reliable rotational constants to guide the assigment of the C1 conformer, while the MP2/cc-pVDZ level fully succeeded.


 Please wait while we load your content...
Please wait while we load your content...