Conformational changes in hydroxyl functional groups upon hydration: the case study of endo fenchol†
Abstract
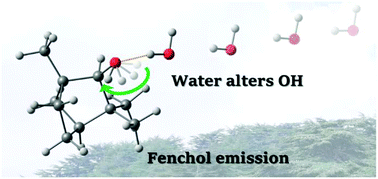
The hydration of endo-fenchol has been studied in the gas phase using a combination of Fourier transform microwave spectroscopy coupled to a supersonic jet expansion and theoretical calculations in the 2 to 20 GHz range. An endo-fenchol⋯water complex was observed. Multi-isotopic substitutions of deuterated species have also been studied in order to confirm the identity of the observed monohydrated endo-fenchol due to the flexibility of the OH group. Herein, the structure of the observed conformer was unveiled. Water induced an alteration in the arrangement of the hydroxyl group. The observed species is stabilized by a hydrogen bond between one water molecule and the highest energy conformer of endo-fenchol, which was not observed in our previous study of the fenchol monomer. This study highlights the flexibility of alcohol molecules and the effect of the strong (O–H⋯O) and weak (C–H⋯O) hydrogen bonds on the stabilization of the cluster with water.


 Please wait while we load your content...
Please wait while we load your content...