The oxidation mechanism of gas-phase ozonolysis of limonene in the atmosphere†
Abstract
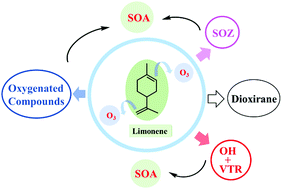
Limonene with endo- and exo-double bonds is a significant monoterpene in the atmosphere and has high reactivity towards O3. We investigated the atmospheric oxidation mechanism of limonene ozonolysis using a high level quantum chemistry calculation coupled with RRKM-ME kinetic simulation. The additions of O3 can take place at both the endo- and exo-double bonds with a branching ratio of 0.87 : 0.13, forming four major highly energized CIs* (named Syn-2a*, Syn-2b*, Anti-2b* and Anti-2c*) with the relative higher fractions of 0.21 : 0.35 : 0.27 : 0.11. A yield of 4% for Limona-ketone was obtained as well. For the unimolecular isomerization pathways of limonene + O3 → POZs → CIs* → SOZ, VHP, or dioxirane, five, one, or none of the internal rotations are treated as hindered internal rotors for CIs*. We obtained percentages of 0.59 : 0.18 : 0.14 in total for separate isomerization routes in the formation of VHPs, dioxirane and SOZs from CIs* using the fourth-order Runge–Kutta method. Additionally, a yield of ∼5% was acquired for stabilized CIs compiling the fractions of different addition routes. About ∼10% of stabilized Anti-2b would isomerize to VHP and 90% would isomerize to SOZs. Isomerization to VHPs dominates the fate of stabilized Syn-2a, Syn-2b and Anti-2c. The overall yield of OH radicals was 0.61. Our study suggested a yield of 0.17 for stabilized SOZs and 0.18 for dioxirane, although both their fates are ambiguous.


 Please wait while we load your content...
Please wait while we load your content...