Low-temperature Raman spectroscopy of sodium-pump rhodopsin from Indibacter alkaliphilus: insight of Na+ binding for active Na+ transport†
Abstract
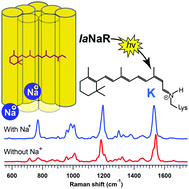
We carried out the low-temperature Raman measurement of a sodium pump rhodopsin from Indibacter alkaliphilus (IaNaR) and examined the primary structural change for the light-driven Na+ pump. We observed that photoexcitation of IaNaR produced the distorted 13-cis retinal chromophore in the presence of Na+, while the structural distortion was significantly relaxed in the absence of Na+. This structural difference of the chromophore with/without Na+ was attributed to the Na+ binding to the protein, which alters the active site. Using the spectral sensitivity to the ion binding, we found that IaNaR had a second Na+ binding site in addition to the one already specified on the extracellular surface. To date, the Na+ binding has not been considered as a prerequisite for Na+ transport. However, this study provides insight that the protein structural change induced by the ion binding involved the formation of an R108–D250 salt bridge, which has critical importance in the active transport of Na+.


 Please wait while we load your content...
Please wait while we load your content...