Radical pair formation due to compression-induced electron transfer in crystals of energetic salts†
Abstract
An interesting effect was observed when studying explosive and non-explosive crystalline ionic materials at high pressures. A wide benchmark set of 76 crystals of different families was studied using the state-of-the-art methods at ambient pressure and in extremes (at 20, 50 and 100 GPa). It was found that hydrostatic compression leads to an electron transfer from the anion to the cation, which was carried out with different efficiencies for explosive and non-explosive salts. The measure of this electron transfer is reflected in the Hirshfeld charges (q) on cations, which decreased with the rise of pressure. Non-explosive materials are generally resistant to this effect, while explosives are much more susceptible. Thus, at 100 GPa, all the studied energetic salts demonstrate qcat < +0.1e, while for the non-explosive salts qcat > +0.1e. This value can be considered as a conditional boundary between explosive and non-explosive salts. The observed effect is in accord with the Szigeti's dielectric theory as well as with the electrophilicity/electronegativity equalization principle. In the present paper, we develop a mechanism of the explosive decomposition based on the assumption about formation of a radical pair as a result of the following reaction: 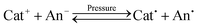 . The study of such radicals revealed their intrinsic instability, which generally reflects either in a dissociative structure or in the presence of strongly weakened trigger bonds.
. The study of such radicals revealed their intrinsic instability, which generally reflects either in a dissociative structure or in the presence of strongly weakened trigger bonds.



 Please wait while we load your content...
Please wait while we load your content...