Stable nitrogen-rich scandium nitrides and their bonding features under ambient conditions†
Abstract
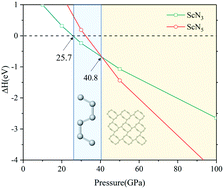
All-nitrogen salts have attracted extensive attention because of their unique chemical, physical properties, and potential applications as high-energy density materials. Using first-principles calculations and a particle swarm optimization structure search method, the pressure versus composition phase diagram of the Sc–N system is established. A new stable phase of C2/m-ScN5 with the intriguing 2D N106− is identified for the first time and we also found ScN3 with the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) structure which has been reported before. Under ambient conditions, both of them have high kinetic and thermodynamic stability with high energy density (2.40 kJ g−1 and 4.23 kJ g−1 relative to ScN and N2 gas). The analyses of chemical bonding pattern indicate that the nitrogen atoms in the N66− chains are connected by covalent bonds with a combined σ and π bond character. We also give a possible high-pressure experimental route to P
structure which has been reported before. Under ambient conditions, both of them have high kinetic and thermodynamic stability with high energy density (2.40 kJ g−1 and 4.23 kJ g−1 relative to ScN and N2 gas). The analyses of chemical bonding pattern indicate that the nitrogen atoms in the N66− chains are connected by covalent bonds with a combined σ and π bond character. We also give a possible high-pressure experimental route to P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) -ScN3 and C2/m-ScN5 at modest pressure. We expect that our theoretical research could encourage experimental realization in the future and contribute to the understanding of the bonding features of nitrogen chains.
-ScN3 and C2/m-ScN5 at modest pressure. We expect that our theoretical research could encourage experimental realization in the future and contribute to the understanding of the bonding features of nitrogen chains.


 Please wait while we load your content...
Please wait while we load your content...