A first-principles study of the atomic layer deposition of ZnO on carboxyl functionalized carbon nanotubes: the role of water molecules†
Abstract
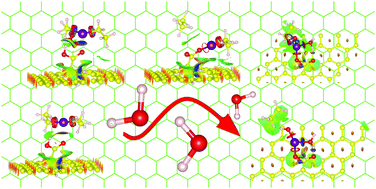
The formation of heterostructures that combine a large surface area with high surface activity has attracted the attention of the scientific community due to the unique properties and applications of these heterostructures. In this work, we describe – at the atomic level – the full reaction mechanisms involved in the atomic layer deposition of a hybrid ZnO/CNT inorganic structure. First, the pristine CNTs are chemically activated with a carboxylic acid, a process unique to carbon materials. Diethylzinc (DEZ) and water are used as gas-phase precursors to form ZnO. Our findings show that DEZ is physically adsorbed on the CNTs during the exposure of the first precursor. The ligand-exchange to generate chemisorbed ethyl zinc on the O side of the COOH group needs to overcome an energy barrier of 0.06 eV. This is a very small energy if compared to the values (0.5–0.6 eV) obtained in previous studies for OH functionalized surfaces. The height of the barrier is associated with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O side, which mediates the H proton's exchange from the OH group to the C2H5 ligand. Furthermore, upon exposure to the oxidizing agent (H2O), ethyl zinc exchanges its last ligand as ethane, and it accepts a hydroxyl group through a self-limiting reaction with an energy barrier of 0.88 eV. Notice that the energy barrier of the second ligand-exchange is larger than of the first. We have also analyzed the effect in the saturation of the second precursor: as the quantity of water molecules increases, the long-range interactions tend to repel them. However, the energy barrier of the second ligand-exchange decreases from 1.53 eV to 0.88 eV for one and two water molecules, showing a clear dependence on the oxidizing agent. Non-covalent interactions are used as a tool to visualize the driving forces that take place during each partial reaction in real space. Our study points out the importance of using the right functionalization agent to achieve a controlled and conformal ALD growth at the initial steps of the formation of hybrid ZnO/CNT structures, as well as the role played by the oxidizing agent to lower the energy barrier on the second ALD step.
O side, which mediates the H proton's exchange from the OH group to the C2H5 ligand. Furthermore, upon exposure to the oxidizing agent (H2O), ethyl zinc exchanges its last ligand as ethane, and it accepts a hydroxyl group through a self-limiting reaction with an energy barrier of 0.88 eV. Notice that the energy barrier of the second ligand-exchange is larger than of the first. We have also analyzed the effect in the saturation of the second precursor: as the quantity of water molecules increases, the long-range interactions tend to repel them. However, the energy barrier of the second ligand-exchange decreases from 1.53 eV to 0.88 eV for one and two water molecules, showing a clear dependence on the oxidizing agent. Non-covalent interactions are used as a tool to visualize the driving forces that take place during each partial reaction in real space. Our study points out the importance of using the right functionalization agent to achieve a controlled and conformal ALD growth at the initial steps of the formation of hybrid ZnO/CNT structures, as well as the role played by the oxidizing agent to lower the energy barrier on the second ALD step.


 Please wait while we load your content...
Please wait while we load your content...